Controlling differentiation and proliferation in human stem cells intended for therapeutic use

OTOSTEM developed two strategies using human stem cells for curing hearing loss: 1) stem cell-derived cellular agents for cell-based therapy and 2) stem cell-derived assays as a screening tool to identify novel drugs. To be safe and effective for therapy, transplantable human otic stem cells must be reliably produced in both purity and quantity. Novel procedures for such high pure production need to be developed. Likewise, identification of drug candidates requires extensive in vitro and in vivo investigations. OTOSTEM intends to bring new therapies to the point of entering a pre-clinical developmental stage in order to prepare grounds for clinical development.
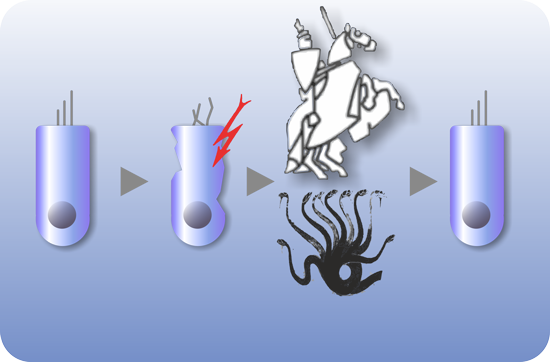
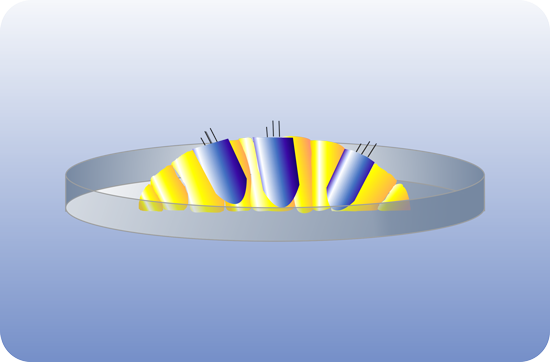
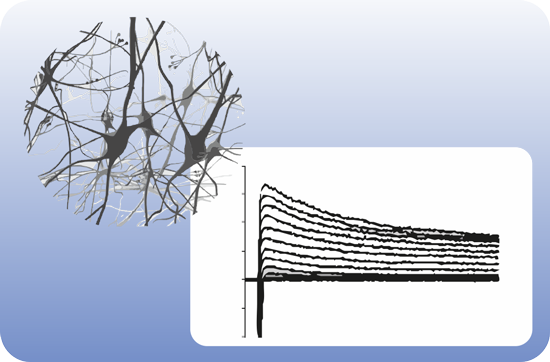
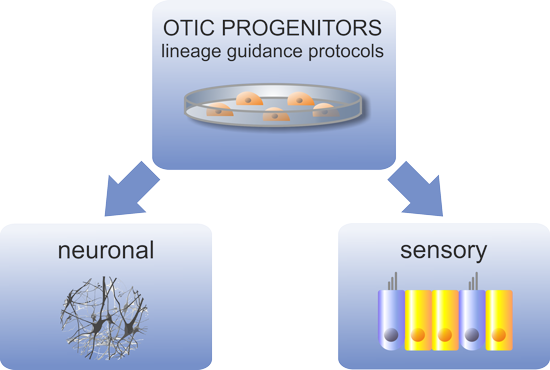
OTOSTEM has identified the principal technological hurdles for the development of cell-based and drug-based hearing loss therapies and addressed these hurdles in successive and systematic manner. Otic progenitor populations were differentiated into cochlear cell types such as sensory cells, supporting cells and neurons. The cross validation from different stem cell sources (hESCs, hiPSCs, native human fetal and adult inner ear stem cells) represented a unique opportunity and particular strength of the OTOSTEM consortium that will provide an unprecedented potential for optimization and selection of the most suitable human stem cell protocols to generate otic progenitors and differentiated cells.